Studying Electron Transfer Pathways in Oxidoreductases
REVIEW
Abstract
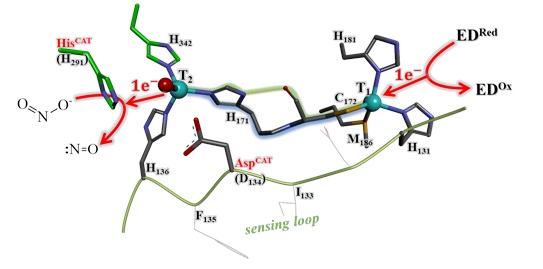
Oxidoreductases containing transition metal ions are widespread in nature and are essential for living organisms. The copper-containing nitrite reductase (NirK) and the molybdenum-containing aldehyde oxidoreductase (Aor) are typical examples of oxidoreductases. Metal ions in these enzymes are present either as mononuclear centers or organized into clusters and accomplish two main roles. One of them is to be the active site where the substrate is converted into product, and the other one is to serve as electron transfer center. Both enzymes transiently bind the substrate and an external electron donor/acceptor in NirK/Aor, respectively, at distinct protein points for them to exchange the electrons involved in the redox reaction. Electron exchange occurs through a specific intra-protein chemical pathway that connects the different enzyme metal cofactors. Based on the two oxidoreductases presented here, we describe how the different actors involved in the intra-protein electron transfer process can be characterized and studied employing molecular biology, spectroscopic, electrochemical, and structural techniques.