Membrane-peptide interaction: Focusing on membrane properties
REVIEW
Abstract
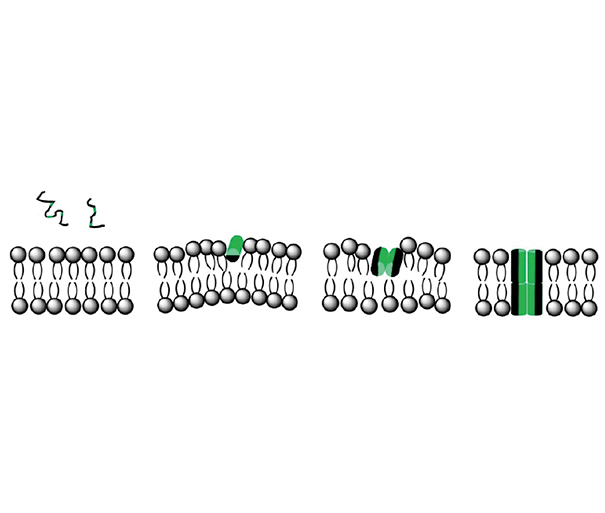
Cellular membranes compartmentalize cells, comprise a permeability barrier, and are the starting place for several signaling cascades and processes in which lateral diffusion of molecules is a key factor. Although it has been shown that organisms adapt the lipid composition of their membranes in order to maintain these in a mainly fluid state, several studies point to the coexistence of regions with different compositions and mechanical properties. In this context, while proteins have been related to solid docks, sterols are accepted as liquid-ordered phase state inducers. Thus, the current model for membranes is a patchwork-like surface, with the different regions being highly variable in size and very dynamic.
Many peptides, like cationic antimicrobial peptides and cell penetrating peptides, target cell membranes. The affinity of these soluble peptides to membranes depends on membrane features such as composition, charge density, compaction, and fluidity. As a consequence of the patchwork-like character of the membrane, regions with a broad spectrum of properties are available to interact with these peptides. Therefore, it is important to know how peptide-membrane interaction depends on membrane properties, and also what happens with the membranes after the interaction.
Here, we summarize our contribution to understanding how the interaction of peptides with membranes is modulated by membrane properties. The influence of the phase state, electrostatics, and chemical composition of the membrane on peptide binding is described using biomimetic systems. The effect of peptide association on membrane properties is also revisited. Finally, possible extrapolations to cells are discussed.